In 1971 when Richard Nixon launched our nation …
into a war on cancer, little did we realize that this war would have significant collateral damage. Although we have made progress against certain types of tumors, the part of the battle that involves wide-spread screening of healthy adults has been a very mixed blessing indeed. This article discusses the challenges and downsides of screening perfectly healthy, asymptomatic, average risk patients for cancer.
Cancer screening really began with the Pap test, named for a Greek pathologist, George Papanicolaou (Pap’’-a-nickle’-aue). Originally it was called a “Pap smear” because the technique included smearing the specimen from the cervix on a slide. Now-a-days, we often use a brush and just drop it in preservative, so there is no smearing and Pap test is the preferred term. Papanicolaou’s likeness was on the reverse side of the Greek 10,000 drachma (worth about $40) before Greece switched to the Euro, but he may be back in circulation soon.
Pap testing reduces the risk of cervical cancer by at least 80% and even up to 99% in some populations. If the Pap test could reduce the risk of cervical cancer so dramatically, certainly other screening tests could do the same for other cancers. Or could they?
In fact, screening healthy people for any disease is highly problematic. When a screening test comes up as positive for cancer, it could be a true positive, meaning that the patient really has cancer, or a false positive, meaning that the test is wrong and the patient does not have cancer. With millions of healthy people out there, even a very low false positive rate is going to generate many thousands of false positive results. Consider the math: if 1% of the population has a cancer and we screen everyone with a test that has a 2% false positive rate, then two-thirds of the positive results are false positives. This can lead to a lot of unnecessary biopsies.

For many types of cancer, including that of the lung, breast, colon, prostate, and kidney, the key bio-logical feature which separates benign (non-cancerous) growths from malignant ones is the capability of the tumor to undergo metastasis. Metastasis means the spread of cancer to other parts of the body. If a pathologist looks at a biopsy specimen under the microscope and sees abnormal cells that resemble the cells we’ve studied in patients with metastatic cancer over the past 100 years, he or she concludes that the specimen in question is malignant. The patient has cancer.
Early in the course of many curable cancers, cells from the tumor circulate in the bloodstream. In animal studies, 99.99% of these cells are incapable of growing any-where outside of the original tumor site. So the problem is not that cells break off and circulate, it is that they ac-quire the ability to attach and grow in a different organ. If you analyze the blood of a cancer patient using very sophisticated laboratory techniques, you will find between one and ten tumor cells in every milliliter of blood. There are a billion red blood cells in that same milliliter, so it is a needle-in-haystack phenomenon. But looked at another way, one or two tumor cells per milliliter of blood means 5,000–10,000 circulating cancer cells even in a patient with an excellent prognosis. Cells from healthy organs, such as kidney and liver, also float around the circulation, but of course can never grow elsewhere.
In most tumors, only a tiny fraction of all the cancer cells ever acquire the ability to metastasize. That a few cells out of a billion develop this ability represents the dark underbelly of the Darwinian algorithm, the same algorithm that allows life itself to exist on our planet. Tumor cells live in a challenging environment. They are often semi-starved for oxygen because tumor growth outpaces the blood sup-ply. Also, our bodies may be attacking the tumor so there are often white blood cells and chemicals present trying to kill off the malignancy. As a result, the billions of cancer cells that are growing and dividing are under attack. Since one feature of cancer is a high mutation rate, the population of cells is quite heterogeneous, just like the human population. We’ve evolved the ability to withstand many infectious diseases that try to kill us, and we evolved the ability to survive in environments far from our origins as tree-dwelling primates in Africa. Likewise, cancer cells sometimes evolve the ability to evade our bodies’ defense mechanisms and grow in a foreign site, such as the lung or bone marrow.
And this brings us right back to the dilemma of cancer screening. What if a massive screening program mostly detected stable, very low grade tumors with a tiny chance of spread? Might we end up burdening thousands of patients with a cancer diagnosis and unnecessary treatment? Sure, we’ll save a very few patients’ lives, but at a huge cost to most of the folks who never needed to be treated for cancer in the first place. On the other hand, what if our screening only identified highly aggressive tumors for which early treatment does not change survival? Now we’ve subjected millions of healthy patients to a screening test that does them no good.
The situation with PSA testing to screen for prostate cancer is illustrative. During 1975, prior to the onset of screening, we identified about one case of prostate cancer for every 100 white males in the United States. Nowadays, the rate is 1.5 per 100 men, owing to widespread PSA testing.
The good news is that the mortality from prostate cancer has fallen by 30%, likely from both screening and improved treatments. So, what’s not to like?
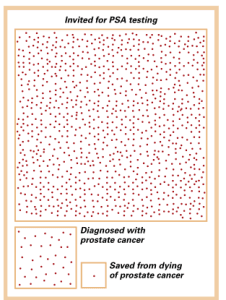
In order to save one man from dying of prostate cancer, we need to invite 1,000 men to be tested every 2-4 years for over a decade and treat the 37 cancers we discover. We still don’t help overall life expectancy. Also, we have not exactly been heroes to the other 36 guys who had a cancer treatment that was of no benefit to them, although the urologists don’t want to admit it.
Well, the problem is that screening for prostate cancer is very good at picking up low grade tumors. Al-though the risk of a doctor writing “prostate cancer” on the death certificate has gone down, the life expectancy of the men who were screened and treated did not change much. Many of the men who used to die of prostate cancer were old and frail and if not for the prostate cancer would have died of something else around the same time. Also, our prostate cancer treatments killed or weakened some men, decreasing the overall benefit of screening. Some screened patients were shown to have very aggressive cancers and ended up with prostate surgery or radiation which was of no benefit to them since the cancer had already metastasized on a microscopic basis before treatment. In all the research trials, no one ever showed a prolonged life expectancy in men with prostate cancer who were treated beyond age 65 because most forms of this cancer grow very slowly.
Physicians who treat prostate cancer have grown more sophisticated. Nowadays, for certain men the recommendation is not to treat low grade cancers but just monitor them. Well, should we really call it a “cancer” in such a situation? Can you imagine going in a time machine to 1955 and telling a patient, “We’ve diagnosed you with cancer and we’re just going to monitor it.” They would think you were nuts.
If urologists are beginning to back off of treating some prostate cancers, public health types are pushing back even more. The United States Preventative Service Task Force (USPSTF) is a government-funded agency that attempts to provide unbiased recommendations on screening tests. Their new draft opinion on PSA argues that we should give up PSA screening al-together. Their conclusion is that no study has ever shown that screening for prostate cancer at any age prolongs life expectancy, even if it does reduce the risk of dying of prostate cancer versus other causes. In addition, 95% of men who have a cancer discovered by PSA screening and who are followed for 12 years do not die of that cancer. The USPSTF makes the point that the diagnosis of very aggressive or very slow moving cancers picked up on screening do not help men at all, and often are harmful, since 5 out of every 1000 men who have prostate surgery die within one month, most often from complications of surgery.
To be fair, others argue that there are substantial methodologic flaws in every PSA screening trial which has ever been conducted. They argue that the Task Force based its recommendations on imperfect scientific data. But still, shouldn’t we, as physicians and scientists, evaluate a screening program with several well-conducted randomized trials before foisting it on the public? Back in 1990, that is what should have been done with PSA testing, instead of turning it into a whole prostate cancer industry.
In my own practice, I have been recommending a less aggressive approach to prostate cancer treatment in my older patients for years. How-ever, for younger men in their fifties and early sixties, I’m not willing just yet to fully give up on screening for prostate cancer, although I respect the decision of a patient who wishes to opt out of the whole process. Patients of mine with a history of prostate cancer who have been treated at places like Swedish, Virginia Mason, or UW should know that by getting care at a top medical center, they probably experienced a lower than average rate of treatment-related side effects and a better overall outcome, so their results may be better than what is seen in some of the large clinical trials. Also, they are certainly less likely to die of prostate cancer and for many patients, not dying of this disease is worth treating it, even if overall life expectancy is not increased. I suspect that if we did a well-conducted study of really healthy, young, vigorous patients with an early diagnosis of prostate cancer from PSA screening, we possibly would find a benefit in terms of overall life expectancy, but we don’t have that study and may never have it. Folks on both sides of the PSA screening debate agree that testing PSA does result in the diagnosis of many early prostate cancers that may never harm a patient.
For breast cancer, we’ve seen a nearly 30% increase in new diagnoses since 1980, and the rising incidence is all due to increased mammographic screening. Some of these cancers might have spontaneously regressed if they were never treated. From one autopsy study of 110 women, average age 39, almost one in five had microscopic evidence of ductal carcinoma in situ, a pre-cancerous change in the breast which, if it shows up on your mammogram, is going to get you a lumpectomy and radiation. Clearly, if all those lesions went on to cancers, the incidence of breast cancer would be substantially higher. Other studies suggest that two-thirds of these precancerous lesions never turn in to breast cancer. In the same study, among women at an average age of 67 who died from unrelated causes, 8% had an undiagnosed breast cancer. At whatever rate that cancer was or was not progressing, these women died of other causes first.
A study published in April 2012 from the Harvard School of Public Health concludes that 20% of all breast cancer diagnoses from mammography are of tumors which never would have caused any harm. The authors state, “over diagnosis and unnecessary treatment of nonfatal cancer creates a substantial ethical and clinical dilemma and may cast doubt on whether mammography screening programs should exist.” What’s worse is that this data came from Norway. In the United States the percentages are probably even higher.
The number of new
cases of melanoma since 1973
has tripled in women.
Another challenge to determining the value of mammography is that the risk of death from breast cancer has fallen by 34% since the largest mammography trials were conducted. How much of this decline is due to screening versus the revolution in breast cancer treatment? Some would argue that with the gains made in treating breast cancer, the value of early diagnosis from mammography has gone down. No one knows for sure.
Malignant melanoma is another terrifying cancer worthy of reconsideration. The popular press often reports that “the incidence of melanoma is increasing.” We’ve all been lying around in tanning booths, heading to the beach with baby oil, depleting the ozone layer, etc, and our come-uppance is a massive increase in this highly aggressive cancer. The number of new cases of melanoma since 1973 has tripled in women. But despite this massive increase, the number of deaths from melanoma in this group has not really changed at all in 30 years. One very charitable interpretation is that, thank goodness, we doctors are removing so many early melanomas that we’ve kept the death rate low despite this massive increase in cancer. A more realistic reading suggests that most of the increase in melanoma is due to removing thin, early melanomas which are of no consequence to the patient and may never have been of any consequence. To be fair, the increase in melanomas in older white males has been associated with a small increase in the risk of death from melanoma, so I can’t argue that all of the increase in melanoma diagnosis has been from detecting harmless lesions. 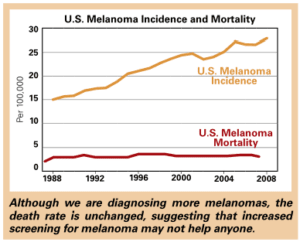
The underlying problem is clear: doctors and patients get worried about a particular type of cancer, we widely employ a screening test for healthy, asymptomatic patients, and the result is a huge increase in the number of new cancers diagnosed without necessarily impacting life expectancy. For the few patients saved by these tests, there are many more who accrue no benefit or are even harmed. It is a tip-of-the-iceberg phenomenon. In the old days, we only diagnosed cancer when it popped up above the waterline. We diagnosed prostate cancer when patients presented with pain in their bones or were unable to urinate. We diagnosed breast cancer when a lump could be felt by the patient. We diagnosed melanoma when the patient said, “I’m worried about this mole.” But physicians and patients did not fully realize that there is plenty more iceberg, and many, but not all, cancers discovered below the waterline are substantially less lethal and some may be harmless.
One thing we discovered with the Pap test is this: it is a clinical error to perform this test on a young female too soon after she has started having sex. The human papilloma virus which causes cervical cancer is widespread, and many women who are sexually active will get infected with it, but most of them will clear the virus on their own. A Pap test done six months after starting sexual activity may show abnormalities but revert to normal after two to three years, so not testing women until they have been sexually active for three years (or at age 21 generally) is the new recommendation to reduce the risk of an unnecessary diagnosis of an abnormal Pap that requires follow up procedures on the cervix. Screening with the Pap test led the way in cancer prevention, and now less screening with the Pap test is leading the way in preventing unnecessary treatment.
So this is cancer, reconsidered. Many “cancers” diagnosed from these newer, widespread national screening programs are actually tumors of little consequence. These cancers are mixed in with a group of aggressive, scary, and often lethal cancers. We used to treat all these malignancies in the same way. Nowadays, when a cancer or even a pre-cancerous diagnosis is made, patients and physicians must try to distinguish between aggressive tumors which are truly life-threatening and those cancers which we may still treat but often are not lethal and may be of relatively little medical con-sequence to patients.