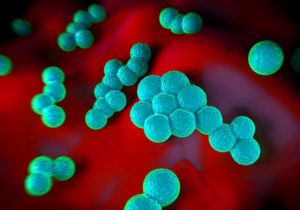
“What is a super bug and how do you …” make one? The term “super bug” always reminds me of the 1972 Black exploitation film Super Fly, starring Ron O’Neil. The movie’s trailer featured the tagline, “He’s got a plan to stick it to the man!”
Back then, there weren’t any super bugs, just Super Flies, but in the ensuing 35 years, antibiotics have been prescribed and over-prescribed so often that these bacteria are now with us for good, and do they ever have a plan to stick it to the man.
A super bug i
The most prevalent super bug these days is Methicillin Resistant Staphlococcal Aureus, or MRSA (pronounced “mer-sa” to rhyme with bursa, curse-a, or purse-a). Staph aureus is a common bug which is pretty much found everywhere—hands, table tops, doorknobs, and so forth. Staph aureus exists on normal skin, along with many other staph species and other bacteria. These organisms exert beneficial effects. It is not proper to have sterilized skin. We need “good” bacteria on our skin, in our gut, and elsewhere to be healthy.
Normally, staph aureus is easily killed by a common penicillin-type antibiotic called methicillin, which has been around since 1959. Interestingly, doctors no longer prescribe methicillin, but it is still used in the laboratory to check for resistance. We use a cousin to methicillin called dicloxacillin instead. A mutation in some strains of staph aureus provides resistance to methicillin, hence the name methicillin resistant staph aureus or MRSA. Bacteria such as MRSA which are resistant to methicillin are, by extension, resistant to all drugs in the penicillin and cephalosporin family. That’s a lot of resistance.
MRSA comes in two varieties, community-acquired, affecting free living folks, and hospital-acquired, which is associated with being in, or having recently been in, the hospital. The hospital-acquired version of MRSA is also resistant to several other antibiotics because most bacteria that live in hospitals have additional antibiotic resistance.
Long before the modern antibiotic era, bacteria were being killed off by antibiotics. Today, you can get penicillin from Rite Aid, but millions of years before its “discovery,” the antibiotic penicillin was actually produced by a common bread mold, penicillium. The mold produces penicillin and also makes bread pretty unappetizing.
When Alexander Flemming, a notoriously sloppy microbiologist, came back to his lab from an extended vacation in 1928, mold was growing in all of his bacterial culture dishes, so he threw them in a sink full of disinfectant. Later, a visitor came by to see his research and Flemming pulled out one culture dish that was not quite submerged in disinfectant. He noticed an unusual pattern: instead of a uniform growth of staph aureus in the dish, in the places where mold was present, there was a ring of clear space and no staph. Louis Pasteur once said, “Chance favors the prepared mind,” and Fleming quickly realized that the mold must be producing something that kills off or inhibits the staph bacteria.
Unfortunately for humanity, Fleming was a lousy chemist, having trained as a physician and microbiologist, and what he needed was a chemical engineer to figure out how to make this molecule of penicillin in large quantities with a stable shelf life. Although he tried to promote interest in penicillin, chemists did not get involved until the 1940’s.
When penicillin was first introduced, it was a highly effective antibiotic and killed all sorts of bacteria. It was invaluable in WWII for battle wounds, for example. It really ushered in the modern era of medicine.
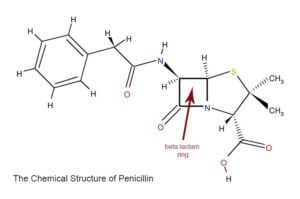
Penicillin, and all antibiotics in the same family including penicillin-like drugs such as dicloxacillin, oxacillin, amoxicillin, and cephalosporins such as Keflex, Vantin, and cefuroxime work because they have a ring called the beta lactam ring, which is a group of three carbon atoms and one nitrogen atom. This is the business, or killing end of the molecule. Other parts of the molecule determine how long it stays in the body, which bacteria it is best at fighting, and so forth.
The beta lactam ring on the antibiotic attaches to the cell membrane of the bacteria at special sites called “penicillin binding proteins.” You might think that bacteria are pretty stupid for having a molecule that binds to penicillin, kind of like if we had an “Al Qaeda Landing Port” in New York for terrorists. In fact, the real role of “penicillin binding proteins” for the bacteria are to help manufacture the bacteria’s cell wall. Bacteria have an outer membrane, just like we have around all our cells, but then, for good measure, they also build a wall, like the Great Wall of China, around that membrane. In certain bacteria, the wall is actually more like a chain-link fence, and these bacteria are not very susceptible to penicillin. Plants also have a cell wall, by the way, and that is what contributes to their fiber component when we eat them.
When penicillin attaches to what we call a “penicillin binding protein” and what bacteria call a “cell wall manufacturing protein,” the bacteria can’t make a good wall. They fall apart and die. The reason why antibiotics don’t kill us is that we don’t have cell walls. If we did, antibiotics would be fatal to humans.
A billion years ago, when the penicillium mold started making penicillin, a few lucky staph aureus bacteria figured out how to make a chemical which breaks this all-important beta lactam ring apart. With that ring broken, the antibiotic is harmless to the bacteria. These counter measure chemicals produced by the bacteria are called beta lactamases because when a chemist adds the suffix “-ase,” it means “an enzyme which breaks something apart.” By contrast, when a chemist adds the suffix “ace,” it means a hardware store. Bacteria that secrete a beta lactamase are resistant to penicillin. Bacteria often keep the DNA for making these beta lactamases separate from their regular DNA. The separate strands of DNA are called plasmids, and they just float around inside the bacteria. By using plasmids, bacteria can share them with other bacteria by shooting a copy over. The bacterium (singular and pedantic for “bacteria”) tells its neighbor, “Hey, I’ve got a great little snippet of DNA which will render us invincible against penicillin! Try it, you’ll like it!” This is one of the many ways that antibiotic resistance spreads.[/vc_column_text][/vc_column][/vc_row][vc_row][vc_column width=”1/1″ animation=”fadeInLeft”][dt_gap height=”10″][/vc_column][/vc_row][vc_row][vc_column width=”1/1″][vc_column_text]Chemists countered this problem by adding another chemical to penicillin, called clavulanic acid. This chemical is a counter-counter measure which binds to the beta lactamase of the bacteria and inactivates it. The popular antibiotic Augmentin is just a mix of penicillin (the antibiotic) and clavulanic acid (the counter-counter measure) to defeat the beta lactamase.
Another approach is to design or discover an antibiotic where the beta lactam ring can’t be broken by the most common bacterial beta lactamases. This is how cephalosporins like Keflex work. Methicillin is another example of an antibiotic which is “beta lactamase resistant,” meaning that the common bacterial beta lactamases can’t destroy it.
Fortunately for us, we have antibiotics which kill, or at least stun bacteria in other ways, not just by disrupting the manufacture of cell walls. These other antibiotics can be effective against MRSA, but bacteria are constantly evolving new ways to fight our antibiotics and it is a never ending “arms race.” Most strains of MRSA already have resistance to at least a few other antibiotics.
On the positive side, “evil drug companies” are spending billions of dollars to come up with new antibiotics to combat this problem of resistance. If it weren’t for many of these new antibiotics, we’d have some bacteria that simply could not be beaten.
Well, if half of what makes a super bug super is resistance, the other half is virulence. Most bacteria rarely hurt us. For example, another type of staph, called staph epidermatitis, is found normally on everyone’s skin and very rarely causes disease. Staph aureus, on the other hand, has always been of two minds in terms of virulence. It is common on the skin and in the noses of many people without causing disease. And yet, when there are breaks in the skin or a wound or an operation, staph is one of the most common organisms to cause an infection. A bacteria can be resistant to 50 different antibiotics, but unless it causes disease, we are not going to consider it a super bug.
Various types of staph have different virulence genes. One, called pvl, for Panton-Valentine leukocidin, attacks our own white blood cells. It is becoming more common in MRSA strains, and this is one way in which MRSA is becoming a super bug. A different mutation allows staph to digest normal barriers in the body’s tissues and spread more easily. A third mutation makes it easy for staph to attach to human and animal cells.
MRSA infections that folks acquire outside of the hospital
often start with what seems like a spider bite.
The infection progresses to a swollen, red, pus-filled sore.
MRSA infections that folks acquire outside of the hospital often start with what seems like a spider bite. The infection progresses to a swollen, red, pus-filled sore. Sometimes just cutting the sore open and letting it drain is curative. Other times, antibiotics are needed. In rare cases, otherwise healthy children have become quite ill or died from MRSA infections outside of the hospital. In these situations, it has not been the antibiotic-resistance of MRSA which caused a fatality, instead it has been the virulence of the infection. These are strains of MRSA which were able to invade the body and grow in the lungs, joints, blood stream, and so forth. These deaths were due to a combination of new virulence genes that are present in MRSA bacteria and some special, rare susceptibility among a few otherwise healthy people to these infections.
One way in which strains of community acquired MRSA are more virulent than other strains is that they are able to fight back against our immune system. These MRSA strains manufacture proteins that specifically attract staph-fighting white blood cells. When the cells arrive, the proteins make them swell up and die.
Interestingly, the antibiotics most likely to be effective against community acquired MRSA are drugs like Bactrim, tetracycline, and clindamycin. These are old, generic drugs, less heavily promoted by drug companies, and unlikely to be given for bronchitis, sore throats, or other obvious viral infections. In the hospital, MRSA is likely to be resistant to many more antibiotics. Because currently or recently hospitalized patients usually are sick for some reason and have weaker immunity, hospital acquired MRSA is more likely to cause a serious infection or a fatality.
Over-prescribing of antibiotics is part, but not all of the problem. Over-prescribing comes from three evil forces: hurried physicians not wanting to take the time to explain to a patient why an antibiotic is not appropriate for a virus, patients who become angry and dissatisfied, feeling the doctor is not taking them seriously if they don’t get an antibiotic prescription when they are sick, and malpractice attorneys who create a fearful climate where some doctors hedge their bets by giving patients with viruses antibiotics on the off chance that they actually have the early stages of an unusual bacterial infection. Unfortunately, people die every year from multiply-resistant bacteria because of these three factors.
The rest of the problem is the development of increasing virulence among bacteria. Normally, bacteria and viruses want to mutate to less virulent forms. If their host is dead, they can’t reproduce and spread nearly as effectively. Still, the inside of our bodies is a warm, nutritious, and tempting target for bacteria and could encourage mutations which allow them to grow there and not just on our skin.
When Alexander Fleming discovered penicillin, he soon saw how improper use of this antibiotic would result in resistant strains of bacteria. Nowadays, virtually all staph is resistant to penicillin. Soon, it may be that all staph is also resistant to methicillin and hence, MRSA will be the predominant species of staph aureus. Because bacteria in different species can trade resistance genes, other species of bacteria may become resistant as well.
Better antibiotic resistance and more virulence among MRSA strains are how this super bug plans to stick it to the man. Fortunately, the man’s biomedical research community and big Pharma have some plans to stick it to MRSA, including the development of a vaccine, more appropriate use of antibiotics, encouraging better handwashing in hospitals, and discovering new drugs to fight bacterial resistance.